Without water, there would not be rivers, lakes, seas or oceans. Then, understanding the behaviour of water molecule is an important step to begin understanding the nature and ocean science.
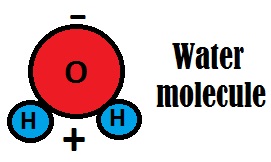
Three atoms, two hydrogen and one oxygen form the water molecule. They are connected by a covalent bond, it means that the hydrogen atoms share electrons with the oxygen atom, forming the water molecule. This configuration makes water a very special substance, an example of this is its fame of being sort of an universal solvent, or a substance that can dissolve everything. This is not 100% true, however, is not wrong to do this statement.
The water molecule has poles of opposite charge (polar molecule), so it can easily dissolve compounds whose elements are connected by means of electrical charges of opposite things as well (e.g., salts), we already talked about this in the post “why the seawater is salty”: https://ourpanthalassa.wordpress.com/2015/04/09/why-is-seawater-salty/. Since the positive part attracts particles with negative charge, its negative part does the opposite, separating and dissolving these compounds. Note the setting of the water molecule below:
This configuration also allows the water to join water molecules, creating a really strong connection between them. When the positive portion of a hydrogen (contained in one molecule of water) is attracted by the negative part of the oxygen of another molecule, they form a “bridge”, which is called hydrogen bridge and keep the molecules joined together by electrostatic forces. One consequence of this unusual union is the surface tension, a property of water that generates some resistance on its surface, enabling some insects to walk on the surface of rivers, lakes and seas.
The water can be found as a gas, liquid or solid. Each of them have different properties, but can exist at the same time on the same place, this is another very special water characteristic.
Solid: The water solidifies when it falls to a temperature from 0 ° Celsius, when its molecules lose their movement and their hydrogen bonds decrease in size. In the ocean, the result of this process is the formation of icecaps and icebergs, however, we must keep in mind a few things about this process in the ocean. The salt water does not solidify, so the formation of icebergs expels salts during the ice formation, making the water at that location saltier than normal. When water solidifies, its volume increases due to the aggregation of gases inside the water molecules, this is only possible because of the shape of the molecule (hexagonal), when this is the ideal temperature for solidification, that’s why ice cubes are not transparent.
Liquid: The liquid state is the most common state of the water, since in most of the world temperatures are above 0 ° Celsius and below 100 ° Celsius. Only in this temperature range the water remains in the liquid state, in which its molecules have some movements, but always kept together by the hydrogen bonds. Liquid water is vital for the life and natural process we know today, from the reproduction of amphibians to the control of the global temperature.
Gas: Perhaps, this is the strangest state to us, because it is not easy to recognize water vapor, but we have gassy water everywhere. When we boil some water to make a tea for example, we see a smoke coming out of the kettle, this smoke is water in the gaseous state. It is also the case of the clouds waiting ideal conditions to transform gas into liquid water. We obtain gaseous water when heated up to a temperature of 100 Celsius, at that time the hydrogen bonds are broken and each water molecule becomes “free”, with a much higher state of agitation in the solid or liquid. But in fact, gaseous water is called humidity and is normally found in little amounts in the air, it does not depend on high temperatures to go to the air.
Remember, during the processes of change between the PHYSICAL states of water, the molecules remain unchanged, which undergoes change is its agitation and the hydrogen bonds that can be short and rigid (solid state), long and soft ( liquid state) or absent (gas). Also, this text does not address the importance of external pressure to define the states of water, as there are numerous factors that alter these processes, but this is extremely important in carrying out practical studies.




Reblogged this on David Fratantoni's Blog and commented:
Water is literally the most important thing on this earth
LikeLike
Pingback: The ocean is a bunch of rivers! | Ocean Science·